JAMA Study Demonstrates D-Mannose Should Not Be Recommended to Prevent UTIs in Women
Daily D-mannose should not be recommended to prevent future episodes of clinically suspected urinary tract infections in women by healthcare providers. This is the primary outcome of a 598-patient, peer-reviewed, randomized, double-blinded, placebo-controlled study published in The Journal of the American Medical Association (JAMA) on April 4th, 2024.
ATLANTA, June 18, 2024 /PRNewswire/ — Urinary tract infections (UTIs) are a pervasive issue, particularly among adult women, 50%-60% of whom will endure at least one in their lifetime, prompting ongoing research into effective prevention strategies. A recent large-scale, multicenter study published in JAMA Internal Medicine challenges the perceived efficacy of D-mannose, a popular supplement touted for UTI prevention. This article delves into the study’s findings, its implications for clinical practice, and the broader conversation about UTI management and the need to seek and utilize more effective alternatives.
The Study and Its Findings
The UK study was conducted across several medical centers and involved a demographic comprising 598 women with an average age of 58, both premenopausal and postmenopausal. Participants were randomized to receive either D-mannose or a placebo daily. Despite high adherence to the regimen, the primary outcome revealed that D-mannose did not significantly reduce the incidence of recurrent UTIs compared to placebo.
Key points from the study include:
- No Significant Reduction in UTI Recurrence: The findings explicitly challenge the existing perception of D-mannose’s effectiveness in preventing recurrent UTIs.
- Secondary Outcomes: Across both groups, there were no significant differences in the duration of UTI symptoms, antibiotic use, time to next UTI, the number of suspected UTIs, or UTI-related hospital admissions.
- High Incidence Rate: Both the treatment and placebo groups experienced high rates of recurrent UTIs, underscoring the ongoing need for effective prophylactic measures.
Clinical Versus Microbiological Diagnosis
One of the study’s critical aspects was its reliance on clinically suspected UTIs rather than microbiological confirmation. This approach highlights the real-world challenges in diagnosing and managing recurrent UTIs in a primary care setting. Over 60% of the clinical encounters involved urine cultures, which is substantial for a trial of this nature but also points to potential misclassification of up to 40% of UTIs in the study.
The frequent discrepancy between clinical symptoms and microbiological findings complicates UTI diagnosis. Many women experience asymptomatic bacterial colonization without UTI symptoms, while others report symptoms that are not due to a microbiological UTI. This conundrum often leads to the overtreatment of asymptomatic bacteriuria, particularly in elderly populations, where it should generally be left untreated unless specific symptoms are present.
The Drawbacks of D-Mannose
Contrary to its widespread use, D-mannose has shown minimal data supporting its efficacy and is not listed as a recommended option for UTI management by the American Urological Association (AUA), Society of Urodynamics Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and the American Urogynecologic Society (AUGS).
Aside from its ineffectiveness in reducing UTI recurrence, D-mannose may pose risks for certain populations. The supplement is known to induce insulin secretion, which can be problematic for individuals with diabetes or insulin resistance, including those with prediabetes. This factor limits its use and raises concerns regarding its overall safety and appropriateness as a prophylactic treatment.
Conclusions and Future Directions
The study’s meticulous design and comprehensive statistical analysis provide a robust basis for its conclusions. With no significant difference in UTI prevention between the D-mannose and placebo groups, the findings are both sound and clinically applicable, suggesting that D-mannose should not be recommended for women with recurrent UTIs.
Wai Lee, MD, Director of Female Pelvic Medicine and Reconstructive Surgery at the Smith Institute for Urology at North Shore University Hospital at Northwell Health, states, “This study reinforces our role as physicians to guide patients away from costly supplements with little or no benefit,” emphasizing the significance of steering patients towards evidence-based solutions.
Further research is needed to explore other potentially effective prophylactic measures that could better serve this at-risk population, ideally focusing on strategies that align closely with clinical evidence and patient safety.
Proven Non-Antibiotic Alternatives
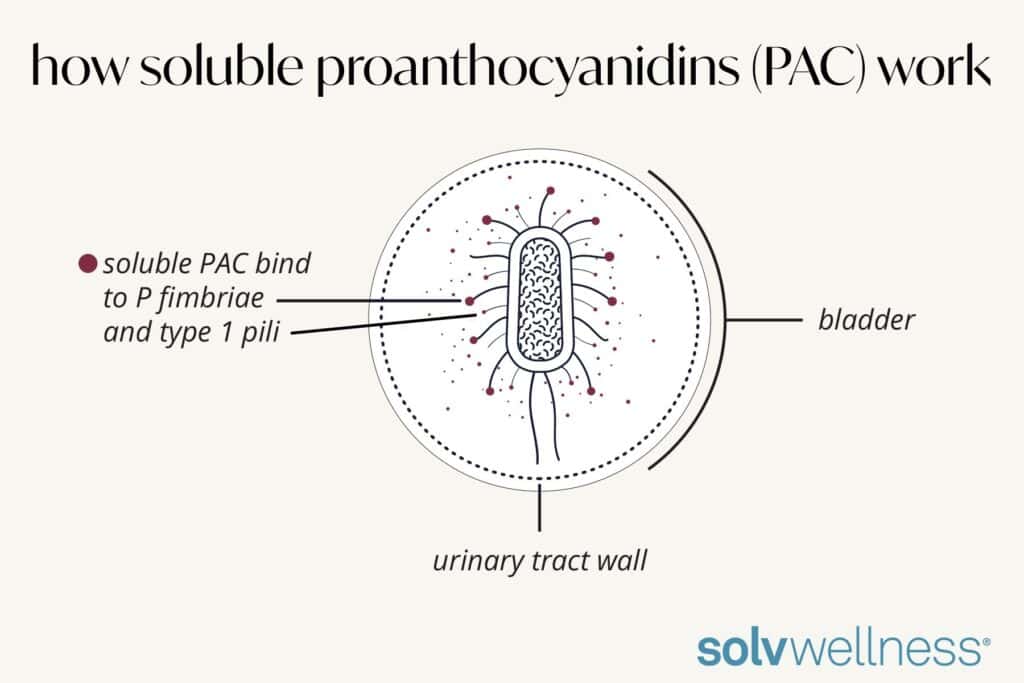
D-mannose is a simple sugar that binds to sugar receptors throughout the body and is mostly absorbed before ever reaching the bladder. If it does reach the bladder, D-mannose only binds to one of the appendages of the infection-causing bacteria: Type 1 pili and not the P fimbriae, the primary causer of UTIs. This is an important distinction, as the adhesion to the P fimbriae is the initial step in causing a UTI, while Type 1 pili simply provokes an inflammatory response prior to the UTI and a mild bacterial anti-adhesion effect.
Proanthocyanidins (PAC) derived from 100% pure cranberry juice extract have been shown to have the highest anti-adhesion activity (AAA) by targeting E. coli and a broader spectrum of uropathogenic bacteria that cause UTIs. By binding to both bacteria appendages, P fimbriae and Type 1 pili, this highly soluble PAC blunts the inflammatory response and aids in the removal of infection-causing bacteria through the urine stream.
Dr. Lee states, “Unlike cranberry supplementation, which is a grade C recommendation by the AUA/SUFU/AUGS recurrent UTI guidelines, there is no consensus on D-mannose. In my practice, I prefer a high-quality cranberry supplement rich in soluble PACs, specifically Ellura.”
Suzette Sutherland, MD, Director of Female Urology at University of Washington, attests to the transformative impact of Ellura in her practice. “The data is just not there to support D-mannose use for UTI prevention,” Dr. Sutherland remarked. “There is plenty of solid science and data behind the use of cranberry supplements as long as they are designed to be potent enough – which means they are made with 100% soluble cranberry juice–based PAC to effectively control the bacteria that cause UTIs.”
As healthcare professionals and researchers continue to advocate for evidence-based solutions in UTI management, Ellura by Solv Wellness stands out as the only cranberry compound on the market today that delivers all the components needed for an effective UTI-preventing supplement: 36mg of 100% cranberry juice–based PAC for improved solubility (bioavailability) and AAA, leading to enhanced effectiveness.
UTI sufferers deserve reliable and scientifically validated approaches to prevent recurrent UTIs. Ellura by Solv Wellness is backed by 21 clinical studies and has 19 traditional medicine approvals worldwide.
Read more about Ellura’s superior efficacy when compared to D-mannose in this Journal of Dietary Supplements study from May 28, 2024.
About Solv Wellness, LLC
Solv Wellness, LLC, delivers products backed by science for often stigmatized pelvic health conditions. For too long, women’s needs at midlife and beyond have been underserved by the scientific and healthcare communities, and as a result, many women often dread much of what aging brings. Solv Wellness is committed to helping bring clarity and confidence to women by providing meaningful solutions, backed by science, to the issues women experience at midlife and beyond. Solv Wellness is the company behind Ellura®, Via™, and Māge™. Ellura is a clinically proven UTI supplement for non-antibiotic management of recurrent urinary tract infections. Via is an FDA authorized 510K nonhormonal vaginal moisturizer made with the highest-quality ingredients that help replenish moisture where women need it most. Māge is a state-of-the-art pre- and probiotic supplement that helps support the entire female pelvic triangle: the gut, vagina, and urinary tract.
For more information about Solv Wellness and its product offerings, visit solvwellness.com.
For scientific data, dosing information, and more, please visit hcp.solvwellness.com.
Contact Info:
Candice Mailman
Senior Marketing Manager
candice@solvwellness.com
+1 (609) 731-9649
Statements in this release have not been evaluated by the FDA.
These products are not intended to diagnose, treat, cure, or prevent any disease.
SOURCE Solv Wellness®
